
Sep 9, 2019 · Historically, probabilistic container closure integrity test methods such as water bath, dye, and microbial ingress tests have been used to determine package quality. These tests are limited in their effectiveness and reliability for several reasons. Chief among them are the subjectivity of the results, the lack of standardization, and the lack

of different components (e.g. bottle, vial, closure, cap, ampoule, blister) which surround the pharmaceutical product from the time of production until its use.' US FDA defines container closure system as 'the sum of packaging components that together contain and protect the dosage form. This includes primary packaging components

Blog. Selecting a Vial Container Closure System with the DeltaCube ™ Modeling Platform. Learn how the DeltaCube™ Modeling Platform helps to facilitate data-driven selection & optimization of vial container closure system (CCS) components in Pharmaceutical Drug Packaging .
Feb 27, 2020 · Introduction. Pharmaceutical product container closure systems (CCS) should be designed to ensure that a drug product (1) is protected from factors that may impact product quality over its shelf life, including light, evaporation, exposure to gases, absorption of water, or microbial contamination; (2) is compatible with the selected CCS and that there is a lack of product—CCS interactions

32060224 10.5731/pdajpst.2019.010843 Compatible vial container closure system (CCS) components in combination with a proper capping process are crucial to ensuring reliable performance, maintaining container closure integrity (CCI), and achieving CCS visual acceptance.

Mar 23, 2020 · The CCS sealing performance inherently will change while undergoing both time and temperature transitions. Exposure to lower temperature has a significant impact on CCS sealing performance, in particular, its container closure integrity (CCI) results. This is critical, as good CCI results are needed to maintain product sterility and stability.
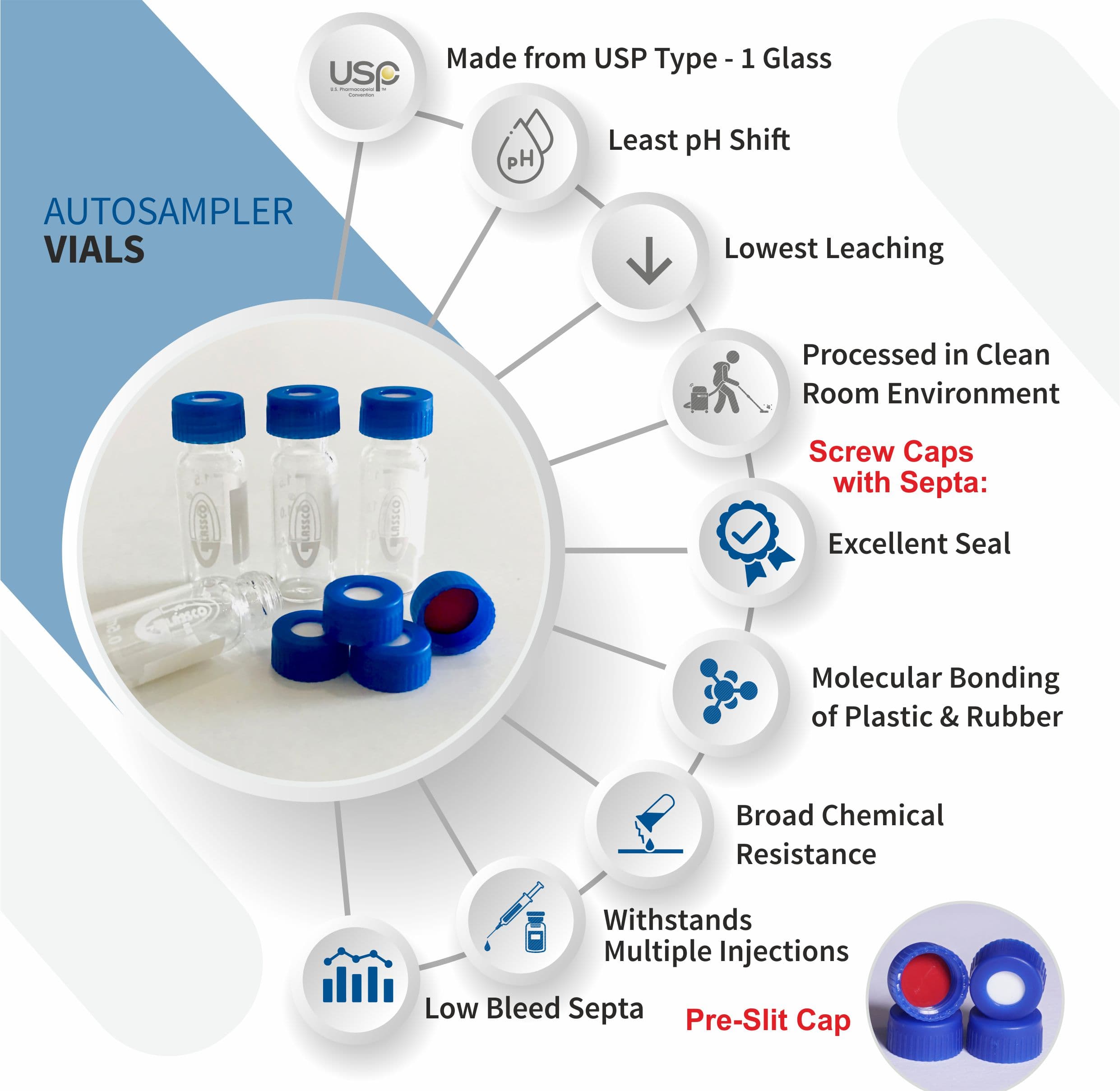
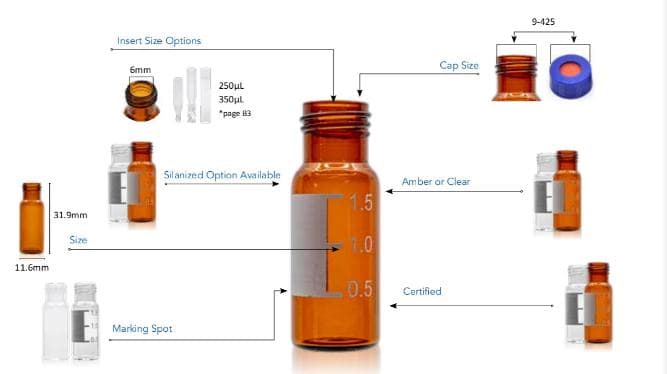
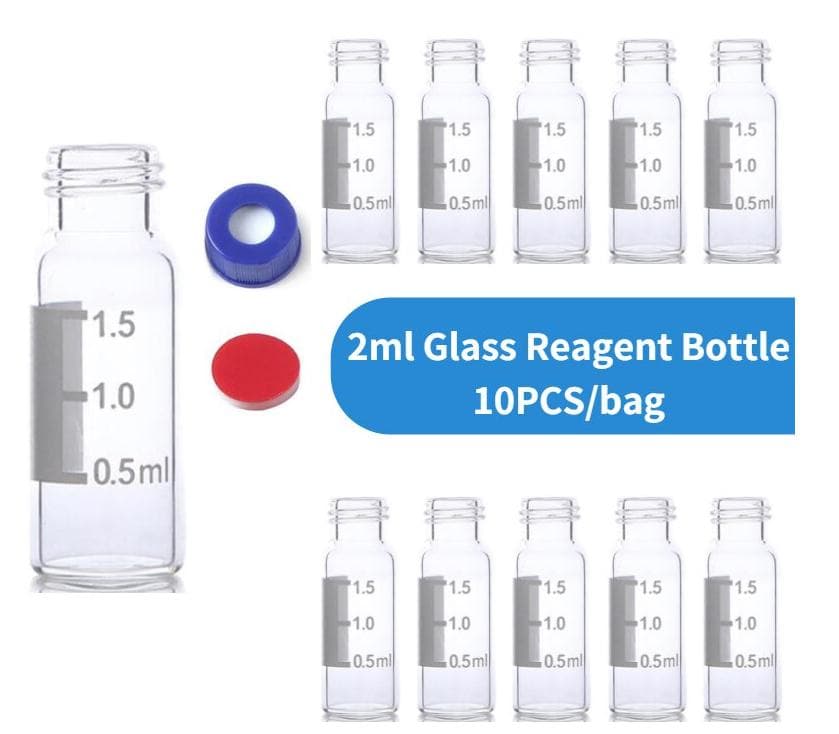
PTFE free, 2 mL storage vial closure. Goal The new Aijiren Tech ™ Chromacol 9 mm screw thread closures, utilizing Advanced Vial Closure System (AVCS) technology, remove obstacles to highly reliable and efficient analysis of samples by offering a complete sample handling solution for chromatography applications in 12 × 32 mm vials

Nov 8, 2021 · As part of developing a control strategy, it is also important to monitor changes in process inputs including excipients and container closure systems. For example, variation in glass vial dimensions may impact heat transfer to the product during lyophilization. Quality attributes unique to the lyophilized products in vials are reconstitution
Advanced Vial Closure System (AVCS) Technology Innovative Closure Technology. Unmatched performance The new AVCS closures are designed to help chromatographers achieve more reliable and efficient sample analysis. Designed to accommodate nearly all chromatography autosamplers fitted for 12x32mm vial trays, these

Feb 23, 2021 · Current trends in the pharmaceutical industry led to a demand for more flexible manufacturing processes with smaller batch sizes. Prepackaged nested vials that can be processed as a unit were introduced into the market to fulfill this need. However, vial nests provide a different thermal environment for the vials compared to a hexagonal packaging array and could therefore influence product

PTFE free, 2 mL storage vial closure. Goal The new Aijiren Tech ™ Chromacol 9 mm screw thread closures, utilizing Advanced Vial Closure System (AVCS) technology, remove obstacles to highly reliable and efficient analysis of samples by offering a complete sample handling solution for chromatography applications in 12 × 32 mm vials

Feb 1, 2016 · The process of the vial closing is a complex interplay of the vial, rubber stopper and crimp cap but also the capping equipment, including the choice of processing parameters and ranges. In this review we describe the challenges regarding CCI and manufacturing defects, such as cosmetic issues associated with the capping process.
Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial contamination, reactive gases (e.g., oxygen), and moisture. A container closure system that permits penetration of microorganisms is unsuitable for a sterile product.

A vial container closure system for pharmaceutical, biological, cell, and gene therapies must maintain container closure integrity to ensure that the drug products remain stable and free of contamination from microbial ingress.